Preformulation: The Key to Successful Drug Development
Table of Contents
Introduction to Preformulation

It is a critical step in the drug development process that involves characterizing the physical, chemical, and biological properties of a new drug candidate. This stage is essential for identifying potential issues and optimizing the formulation of the drug to ensure its safety, efficacy, and stability. Preformulation is a systematic approach that helps to understand the properties of the drug substance, which is crucial for designing a formulation that meets the desired therapeutic and regulatory requirements.
Goals and Objectives of preformulation
The primary goals and objectives :
– Characterize Drug Properties: Determine the physical, chemical, and biological properties of the drug substance, including its solubility, stability, and permeability.
– Identify Potential Issues: Identify potential issues that may impact the drug’s efficacy, safety, and stability, such as poor solubility, instability, or bioavailability issues.
– Inform Formulation Design: Provide data to inform the design of the formulation and ensure the drug is delivered effectively and safely.
– Optimize Drug Development: Optimize drug development by identifying potential issues early on and reducing development time and costs.
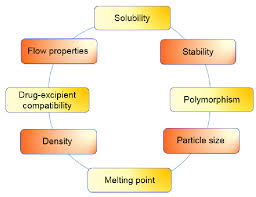
Study of Physicochemical Characteristics of Drug Substances
The study of physicochemical characteristics of drug substances is a critical aspect of preformulation. This includes:
– Solubility: Determining the solubility of the drug in various solvents, including water, buffers, and organic solvents.
– Particle Size and Shape: Characterizing the particle size and shape of the drug, including its morphology and particle size distribution.
– Crystallinity: Investigating the crystalline structure of the drug, including its polymorphism and crystallinity.
– Thermal Properties: Studying the thermal properties of the drug, including its melting point, glass transition temperature, and thermal stability.
– Stability: Evaluating the stability of the drug substance and product, including its chemical stability, physical stability, and microbiological stability.
Importance of Physicochemical Characterization
Physicochemical characterization is essential for:
– Understanding Drug Behavior: Understanding how the drug behaves in different environments and conditions, including its solubility, stability, and permeability.
– Optimizing Formulation: Optimizing the formulation to ensure the drug is delivered effectively and safely, including its bioavailability, efficacy, and safety.
– Ensuring Stability: Ensuring the stability of the drug substance and product, including its shelf life, storage conditions, and packaging requirements.
Techniques Used in Preformulation

Several techniques are used in preformulation to characterize the physicochemical properties of drug substances, including:
– Spectroscopy: Using techniques like IR, NMR, and UV-Vis spectroscopy to characterize the drug’s chemical structure and properties.
– Thermal Analysis: Studying the thermal properties of the drug using techniques like DSC and TGA.
– Particle Size Analysis: Determining the particle size distribution of the drug using techniques like laser diffraction and dynamic light scattering.
– Solubility Studies: Determining the solubility of the drug in various solvents using techniques like shake-flask method and HPLC.
Importance of Preformulation in Drug Development
It plays a critical role in drug development by:
- Informing Formulation Design: The data helps design a formulation that ensures the drug’s safety, efficacy, and stability.
- Reducing Bioavailability Issues: It helps identify potential bioavailability issues and optimize the formulation to improve drug absorption.
- Ensuring Stability: To ensures the stability of the drug substance and product.
Challenges in Preformulation
Despite its importance, It can be challenging due to:
- Limited Drug Supply: It requires a limited amount of drug substance, which can be a challenge for early-stage development.
- Complexity of Drug Properties: Drug properties can be complex and difficult to characterize, requiring specialized techniques and expertise.
- Interplay between Drug Properties: The interplay between different drug properties can be complex, requiring a comprehensive understanding of the drug’s behavior.
flow chart
Conclusion
It is a critical step in the drug development process that helps to ensure the safety, efficacy, and stability of new drug candidates. By understanding the physicochemical characteristics of drug substances, it informs the design of the formulation and identifies potential issues early on. The use of advanced techniques and methodologies in preformulation helps to optimize drug development and reduce development time and costs.
Future Directions
The future of preformulation holds much promise, with advances in:
– High-Throughput Screening: High-throughput screening enables rapid characterization of drug properties and identification of potential issues.
– Artificial Intelligence: Artificial intelligence can help predict drug properties and optimize formulation design.
– Personalized Medicine: It can play a critical role in personalized medicine by optimizing formulations for individual patients.


