Introduction
Parenteral product are sterile preparations administered directly into the body through injection or infusion, bypassing the gastrointestinal tract. These products are designed to provide immediate therapeutic effects, high bioavailability, and targeted delivery. Parenteral products are used to treat a wide range of medical conditions, including infections, cancers, and chronic diseases.
Table of Contents

Types of Parenteral Products
- Intravenous (IV) Products: IV products are administered directly into a vein, providing rapid delivery of medications, fluids, or nutrients. IV products are commonly used in emergency situations, such as cardiac arrest or severe dehydration.
- Intramuscular (IM) Products: IM products are administered into a muscle, providing slower absorption of medications. IM products are commonly used for vaccinations, antibiotics, and pain management.
- Subcutaneous (SC) Products: SC products are administered under the skin, providing slow and sustained release of medications. SC products are commonly used for insulin therapy, hormone replacement, and certain types of chemotherapy.
Advantages of Parenteral Product
- Rapid Onset of Action: Parenteral products provide immediate therapeutic effects, making them ideal for emergency situations.
- High Bioavailability: Parenteral products avoid first-pass metabolism, ensuring high bioavailability and efficacy.
- Targeted Delivery: Parenteral products can be delivered directly to the site of action, reducing side effects and improving therapeutic outcomes.
- Flexibility: Parenteral products can be formulated to provide a wide range of release profiles, from rapid to sustained release.
Limitations of Parenteral Product
- Sterility Requirements: Parenteral products must be manufactured in sterile environments to prevent contamination and infection.
- Cost: Parenteral products can be expensive to produce and administer, making them less accessible to some patients.
- Patient Compliance: Parenteral products require injection or infusion, which can be inconvenient and painful for patients.
- Risk of Adverse Reactions: Parenteral products can cause adverse reactions, such as allergic reactions, infection, or thrombophlebitis.
Preformulation Factors
- Solubility: The drug must be soluble in the vehicle to ensure proper delivery and efficacy.
- Stability: The drug must be stable in the vehicle and during sterilization to maintain its potency and efficacy.
- Compatibility: The drug must be compatible with the vehicle, additives, and container to prevent interactions and degradation.
- pH: The pH of the solution can affect the stability and efficacy of the drug.
Essential Requirements
- Sterility: Parenteral product must be sterile to prevent infection and contamination.
- Apyrogenicity: Parenteral product must be free of pyrogens to prevent fever and other adverse reactions.
- Isotonicity: Parenteral product should be isotonic with blood to prevent hemolysis and other adverse reactions.
- Particulate Matter: Parenteral product must be free of particulate matter to prevent embolism and other adverse reactions.
Vehicles
- Water for Injection: The most common vehicle used for parenteral products, water for injection is sterile and pyrogen-free.
- Oils: Used for depot injections, oils can provide sustained release of medications.
- Cosolvents: Used to enhance solubility, cosolvents can improve the delivery of poorly soluble drugs.
- Other Vehicles: Other vehicles, such as lipids and surfactants, can be used to improve the delivery and efficacy of parenteral products.
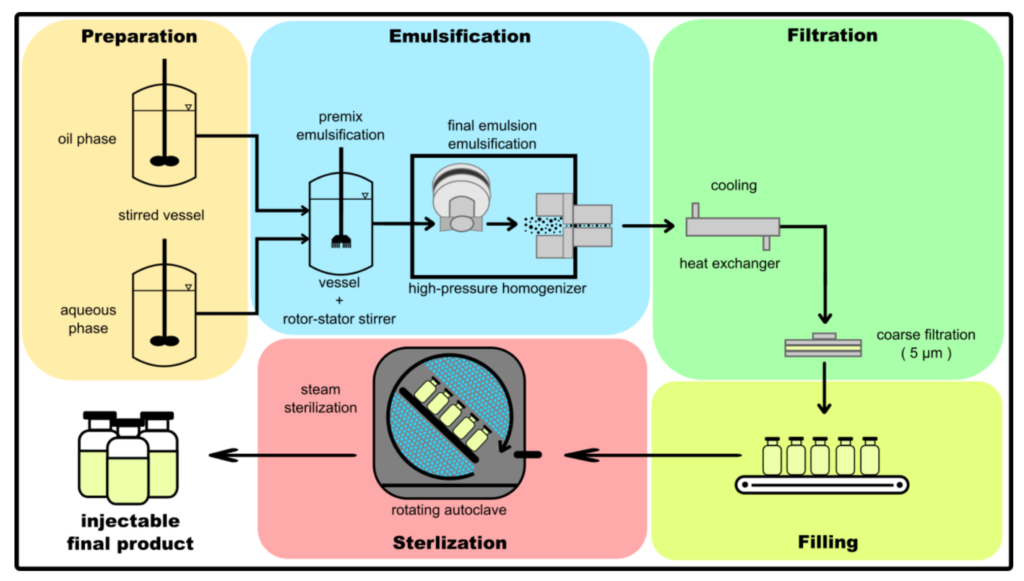
Production Procedure for Parenteral Product
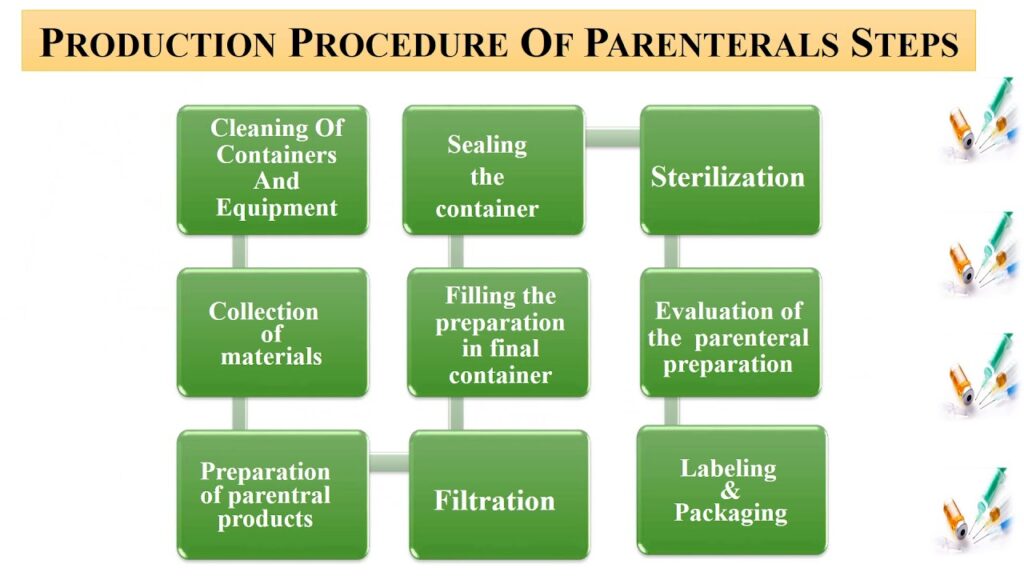
- Formulation Development: Develop the formulation, including the selection of the active pharmaceutical ingredient (API), excipients, and vehicle.
- Sterilization Method Selection: Select the appropriate sterilization method, such as autoclaving, dry heat sterilization, or sterile filtration.
- Batch Preparation: Prepare the batch by mixing the API and excipients in the vehicle.
- Filtration: Filter the solution to remove any particulate matter or contaminants.
- Filling: Fill the sterile containers, such as vials or syringes, with the parenteral product.
- Sealing: Seal the containers to prevent contamination.
- Sterilization: Sterilize the product using the selected method.
- Quality Control: Conduct quality control tests, including sterility testing, pyrogen testing, and particulate matter testing.
- Packaging and Labeling: Package and label the product for distribution.
- Release: Release the product for use after ensuring it meets all quality and regulatory requirements.
Additives
- Antioxidants: Used to prevent oxidation and degradation of drugs.
- Preservatives: Used to prevent microbial growth and contamination.
- Buffers: Used to maintain pH and stability of drugs.
- Other Additives: Other additives, such as tonicity agents and stabilizers, can be used to improve the safety and efficacy of parenteral products.
Importance of Isotonicity
- Prevents Hemolysis: Isotonic solutions prevent red blood cell damage and hemolysis.
- Reduces Pain: Isotonic solutions minimize pain and discomfort associated with injection or infusion.
- Maintains Stability: Isotonic solutions help maintain drug stability and efficacy.
Manufacturing Considerations
- Sterile Manufacturing: Parenteral product require specialized facilities and equipment to ensure sterility.
- Quality Control: Parenteral products require strict quality control measures to ensure safety and efficacy.
- Regulatory Compliance: Parenteral products must comply with regulatory requirements and guidelines.
conclusion
Parenteral products are a crucial class of pharmaceutical preparations that offer rapid onset of action, high bioavailability, and targeted delivery. Despite their advantages, parenteral products require careful consideration of preformulation factors, essential requirements, and manufacturing considerations to ensure safety, efficacy, and stability.
The importance of sterility, pyrogenicity, and isotonicity cannot be overstated, as these factors directly impact the safety and efficacy of parenteral products. Manufacturers must adhere to strict quality control measures and regulatory guidelines to ensure compliance.

