Introduction
Table of Contents
Pharmaceutical aerosols are a type of drug delivery system that uses a propellant to generate a fine spray or foam, allowing for targeted delivery of medications to specific areas of the body. These systems offer several benefits, including improved efficacy, reduced side effects, and increased patient compliance.
Definition and Components
A pharmaceutical aerosol consists of a mixture of a drug, propellant, and other excipients, which are packaged under pressure in a container fitted with a valve. When the valve is actuated, the propellant generates a force that expels the formulation as a spray or foam.
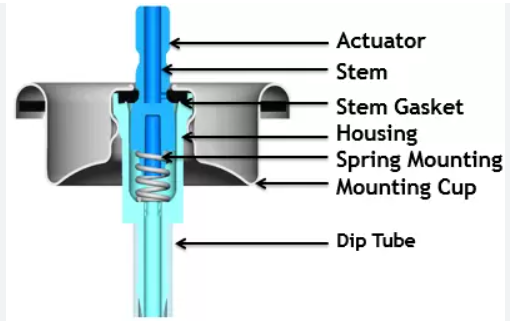
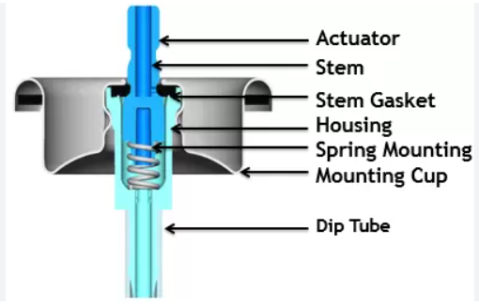
The key components of a pharmaceutical aerosol include:
- Propellants: These are gases or liquids that generate the pressure needed to expel the formulation. Common propellants include compressed gases (e.g., nitrogen, carbon dioxide) and liquefied gases (e.g., hydrofluoroalkanes (HFAs)).
- Container: The container is designed to withstand the pressure generated by the propellant and is typically made of metal, glass, or plastic.
- Valves: The valve controls the release of the formulation and can be either metering or non-metering. Metering valves deliver a fixed dose, while non-metering valves deliver a variable dose.
Types of Aerosol Systems
There are several types of pharmaceutical aerosol systems, including:
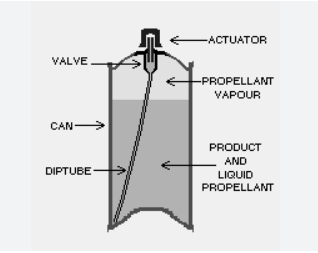
- Solution Aerosols: These systems consist of a drug dissolved in a solvent, which is then mixed with a propellant.
- Suspension Aerosols: These systems consist of a drug suspended in a liquid propellant or a mixture of a propellant and a solvent.
- Emulsion Aerosols: These systems consist of a mixture of two or more liquids that don’t normally mix, such as oil and water.
- Foam Aerosols: These systems generate a foam when the valve is actuated, which can be useful for topical applications.
Formulation and Manufacture
The formulation and manufacture of pharmaceutical aerosols require careful consideration of several factors, including:
- Propellant selection: The choice of propellant will depend on the specific application and the properties of the drug.
- Solvent selection: The solvent should be able to dissolve the drug and be compatible with the propellant.
- Excipient selection: Excipients can be added to improve the stability, solubility, or viscosity of the formulation.
- Mixing and filling: The formulation is mixed and filled into the container under pressure.
- Crimping and testing: The container is crimped to ensure a secure seal, and the aerosol is tested for performance and quality.
Evaluation of Aerosols
The evaluation of pharmaceutical aerosols involves several tests, including:
- Spray pattern and particle size: The spray pattern and particle size can affect the efficacy and safety of the aerosol.
- Dose uniformity: The dose uniformity is critical to ensure that the patient receives the correct amount of medication.
- Valve performance: The valve performance can affect the consistency and accuracy of the dose.
- Microbial testing: Microbial testing is essential to ensure the sterility and safety of the aerosol.
Quality Control
Quality control is critical in the manufacture of pharmaceutical aerosols to ensure their safety, efficacy, and quality. Some of the key quality control tests include:
- Leak testing: Leak testing ensures that the container is sealed properly and that there are no leaks.
- Weight variation: Weight variation testing ensures that the container is filled with the correct amount of formulation.
- Spray pattern testing: Spray pattern testing ensures that the aerosol produces a consistent and accurate spray pattern.
- Microbial testing: Microbial testing ensures that the aerosol is sterile and free from microbial contamination.
Stability Studies
Stability studies are essential to ensure the chemical, physical, and microbiological stability of pharmaceutical aerosols over time. These studies involve testing the aerosol under various conditions, including:
- Temperature: The aerosol is tested at different temperatures to ensure stability.
- Humidity: The aerosol is tested at different humidity levels to ensure stability.
- Light: The aerosol is tested for photostability to ensure that it is not affected by light.
Conclusion
Pharmaceutical aerosols offer a convenient and effective way to deliver medications to specific areas of the body. The formulation and manufacture of these systems require careful consideration of several factors, including propellant selection, solvent selection, and valve design. Quality control and stability studies are essential to ensure the safety, efficacy, and quality of pharmaceutical aerosols.
Q1: What is the primary function of a propellant in a pharmaceutical aerosol?
A1: The primary function of a propellant is to generate pressure to expel the formulation from the container.
Q2: Which of the following is a type of pharmaceutical aerosol system?
A2: Solution aerosol, suspension aerosol, emulsion aerosol, or foam aerosol.
Q3: What is the purpose of a metering valve in a pharmaceutical aerosol?
A3: A metering valve delivers a fixed dose of the formulation.
Q4: What is a critical factor in evaluating the quality of a pharmaceutical aerosol?
A4: Spray pattern and particle size, dose uniformity, or valve performance.
Q5: What is the main advantage of using pharmaceutical aerosols for drug delivery?
A5: Targeted delivery, improved efficacy, or increased patient compliance.
Q6: Which propellant is commonly used in pharmaceutical aerosols?
A6: Hydrofluoroalkanes (HFAs) or compressed gases like nitrogen or carbon dioxide.