INTRODUCTION OF STATES OF MATTER
Table of Contents
The study of states of matter is a fundamental concept in pharmacy, as it plays a crucial role in the development, manufacturing, and quality control of pharmaceutical products. Matter can exist in three main states: solid, liquid, and gas. Each state has its unique characteristics, and understanding these characteristics is essential for pharmacists and pharmaceutical scientists.
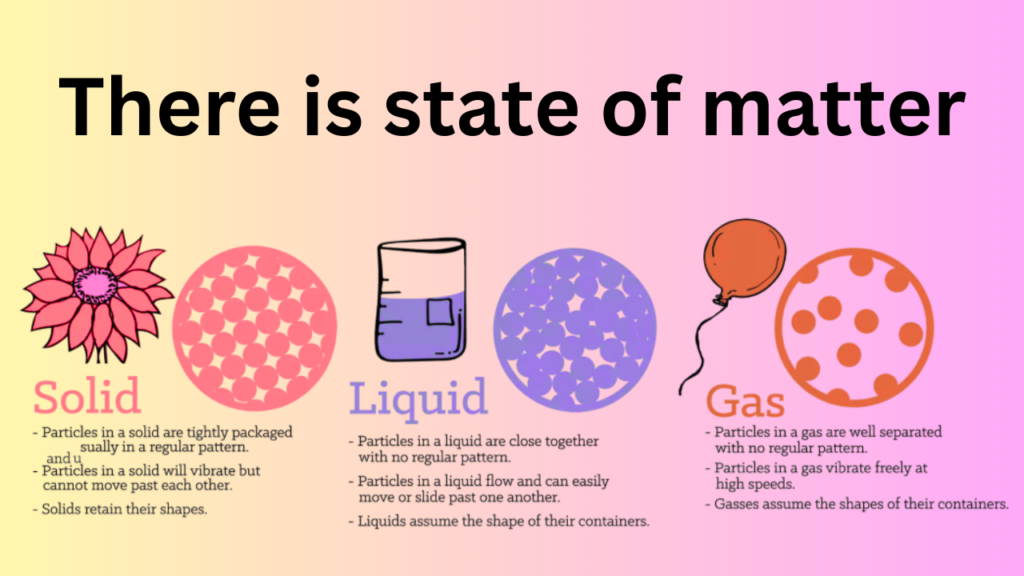
Solids
In states of matter, Solids are substances that maintain their shape and volume. In pharmacy, solids are commonly used in the form of tablets, capsules, and powders. The properties of solids, such as their crystalline structure and solubility, play a critical role in determining their bioavailability and efficacy.
There are several types of solids, including:
- Crystalline solids: These are solids that have a regular, three-dimensional arrangement of molecules. Examples include salts and sugars.
- Amorphous solids: These are solids that lack a regular, three-dimensional arrangement of molecules. Examples include glasses and many polymers.
- Polymorphism: Many substances may exist in more than one crystalline or amorphous form. This phenomenon, where compounds exist in more than one crystalline and amorphous form, is known as polymorphism or polymorphic forms.
- Polymorphism can affect the mechanical properties of drug particles and can therefore affect the manufacturability and physical attributes of dosage forms like tablets. For example, different polymorphic forms of drugs like paracetamol, carbamazepine, phenobarbitone and sulfamerazine have exhibited different mechanical properties such as compressibility, flowability, hardness, bonding strength, etc. Many organic substances such as tristearin and theobroma oil also exhibit polymorphism.
The properties of solids can affect their performance in pharmaceutical products. For example, the solubility of a solid can affect its bioavailability, while its crystalline structure can affect its stability.
Liquids
Liquids are substances that take the shape of their container and have a fixed volume. In pharmacy, liquids are commonly used in the form of solutions, suspensions, and emulsions. The properties of liquids, such as their viscosity and surface tension, are important factors to consider when formulating liquid dosage forms.
There are several types of liquids, including:
- Solutions: These are liquids in which a solute is dissolved in a solvent. Examples include syrups and intravenous solutions.
- Suspensions: These are liquids in which a solid is dispersed in a liquid. Examples include oral suspensions and topical creams.
- Emulsions: These are liquids in which two or more immiscible liquids are mixed. Examples include creams and lotions.
The properties of liquids can affect their performance in pharmaceutical products. For example, the viscosity of a liquid can affect its flowability, while its surface tension can affect its spreadability.
Gases
Gases are substances that have neither a fixed shape nor a fixed volume. In pharmacy, gases are used in various applications, such as anaesthesia and respiratory therapy. Understanding the properties of gases, such as their compressibility and diffusivity, is essential for designing and using gas-based pharmaceutical products.
There are several types of gases, including:
- Compressed gases: These are gases that are stored under pressure in cylinders. Examples include oxygen and nitrogen.
- Anaesthetic gases: These are gases that are used to induce anaesthesia. Examples include nitrous oxide and sevoflurane.
The properties of gases can affect their performance in pharmaceutical products. For example, the compressibility of a gas can affect its storage and handling, while its diffusivity can affect its delivery to the patient.
CHANGE IN THE STATE OF MATTER
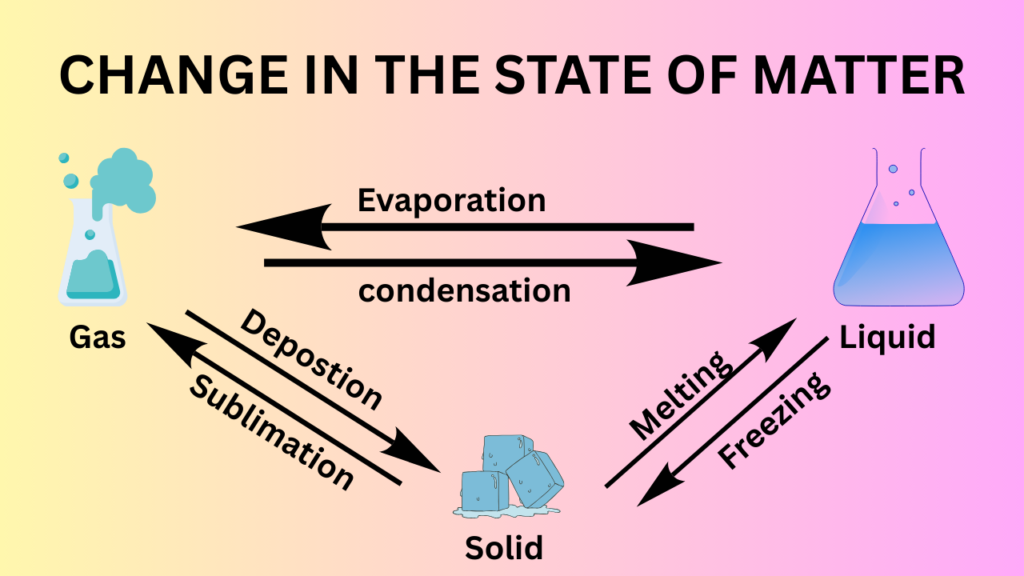
As a solid changes to a liquid state and then to a gaseous state, heat is absorbed and the enthalpy (heat content) of the material increases. Thus, the enthalpy of a liquid is greater than that of a solid, and the enthalpy of a gas is greater than that of the liquid. The entropy (degree of molecular randomness) of the material also increases as it transitions from a solid to a liquid and then to a gas.
Latent Heat
When a change in the state of a material occurs, the temperature usually remains constant, but that heat is absorbed. This heat, which results in the change of matter without increasing the temperature, is called the latent heat. When this heat results in the changes of state from a solid to a liquid, it is known as the latent heat of fusion. For example, the heat required to change ice to water at the latent heat of fusion. Likewise, the latent heat of vaporisation is the quantity of heat absorbed when a change of state from liquid to vapour occurs at its boiling point without changing the temperature of the material. For example, the heat required to change water to vapour at 100 degrees cellcious is the latent heat of vaporisation.
vapour pressure
When a liquid is kept in a closed evacuated container, molecules from its surface continuously leave and gon into the free space above it. This is known as the process of vaporisation. Some molecules, however, return to the surface depending on their concentration in the vapour (the process of condensation). Eventually, a condition of equilibrium is established when the rate of escape of molecules becomes equal to the return.
Phase Transitions
Phase transitions occur when a substance changes from one state of matter to another. In pharmacy, phase transitions are critical in various processes, such as freeze-drying and spray drying. Understanding the thermodynamics of phase transitions is essential for optimising these processes and ensuring the quality of pharmaceutical products.
There are several types of phase transitions, including:
- Melting: This is the transition from a solid to a liquid.
- Freezing: This is the transition from a liquid to a solid.
- Sublimation: This is the transition from a solid to a gas, without going through the liquid phase.
The properties of phase transitions can affect the performance of pharmaceutical products. For example, the melting point of a solid can affect its stability, while its sublimation point can affect its storage and handling.
Applications in Pharmacy of states of matter
Understanding the states of matter has numerous applications in pharmacy, including:
- Dosage form design: Understanding the properties of States of matter in solids, liquids, and gases is critical for designing effective dosage forms, such as tablets, capsules, solutions, and inhalers.
- Pharmaceutical processing: Phase transitions and the properties of states of matter play a crucial role in various pharmaceutical processes, such as crystallisation, filtration, and drying.
- Stability and shelf-life: Understanding the properties of matter is essential for predicting the stability and shelf-life of pharmaceutical products.
In addition, understanding the states of matter is critical for developing and manufacturing pharmaceutical products that meet the required standards of quality, safety, and efficacy.
Conclusion
In conclusion, understanding the states of matter is essential for pharmacists and pharmaceutical scientists. By understanding the properties and behaviour of solids, liquids, and gases, we can design and develop effective pharmaceutical products, optimise pharmaceutical processes, and ensure the quality and stability of pharmaceutical products. The study of states of matter is a fascinating field that has numerous applications in pharmacy, and its importance cannot be overstated.


